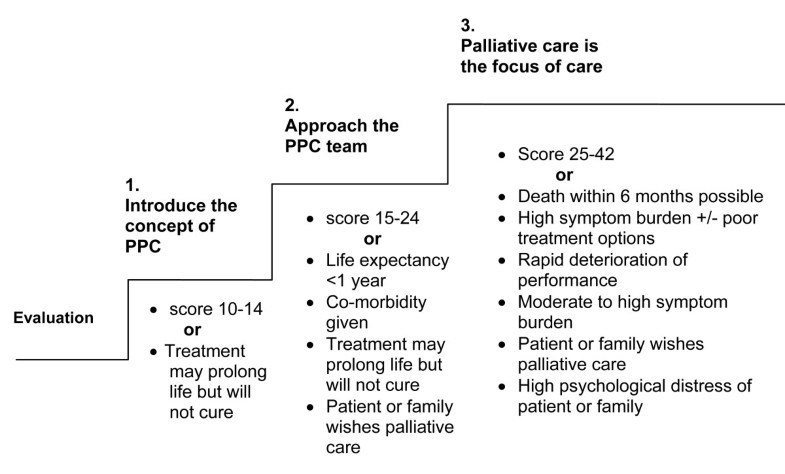
Version 1 of the PaPaS Scale
Domain and Item number
|
Item
|
Characteristic
|
Score (preliminary)
|
Changes made to Version 1
|
|---|---|---|---|---|
Domain 1
|
Estimated life expectancy
|
Position: end of the scale
| ||
1.1
|
Estimated life expectancy
|
> 2 years
|
0 □
|
Qualitative time frames (e.g. weeks to months)
|
> 1 but < 2 years
|
1 □
| |||
3 months to 1 year
|
2 □
| |||
< 3 months
|
3 □
| |||
1.2
|
“Would you be surprised if this patient were still alive in 6 months time?”
|
Yes
|
3 □
| |
No
|
0 □
| |||
Domain 2
|
Expected outcome of current treatment directed at the disease and burden of this treatment
| |||
2.1
|
Expected outcome of treatment directed at the disease
|
There are no treatments currently that can cure the disease or prolong life.
|
4 □
|
Percentages omitted Outcome of treatment described with respect to life extension and quality of life
|
Current treatment patient is receiving or will be receiving may, in only a small number of cases (<20%), prolong life but will not cure.
|
3 □
| |||
Current treatment patient is receiving or will be receiving may, in >20% of cases, prolong life but will not cure.
|
2 □
| |||
Current treatment patient is receiving or will be receiving may cure the illness in <20% of cases.
|
1 □
| |||
Current treatment patient is receiving or will be receiving may cure the illness in >20% of cases.
|
0 □
| |||
2.2
|
Burden of treatments
|
Treatments carry a high level of burden (many side effects).
|
2 □
|
Further explication of ‘burden’ (e.g. side effects, stay in hospital)
|
Treatments carry a low to medium level of burden (few side effects).
|
1 □
| |||
Treatments carry no or minimal burden (side effects) or no treatment is envisioned.
|
0 □
| |||
Domain 3
|
Performance status
|
Rephrased: ‘Trajectory of disease and impact on daily activities of the child’ Position: beginning of the scale
| ||
3.1
|
Current performance status (in comparison with the child’s own baseline)
|
Moderate to severe restriction of play (no active play, requires assistance for quiet play) 0-40% of normal range.
|
3 □
|
Change of characteristics and omission of percentages
|
Mild to moderate restriction of play (able to engage in some active play; requires assistance) 50-70% of normal range.
|
1 □
| |||
Normal range of play (able to carry on usual play activities) 80-100% of normal range.
|
0 □
| |||
3.2
|
Rate of decline of performance status
|
Overall, performance has decreased by half over the last 4 weeks.
|
2 □
|
Time frame and impact of decline included in 3.1
|
Overall, performance has decreased by about a third over the last 4 weeks.
|
1 □
| |||
Overall performance has not deteriorated over the last 4 weeks.
|
0 □
| |||
Domain 4
|
Symptom and problem burden
| |||
4.1
|
Number of symptoms
|
Patient has 3 or more symptoms (e.g. pain, weight loss, fatigue, dyspnoea, nausea & vomiting, depression, anxiety)
|
4 □
|
List of symptoms amended
|
Patient has 2 symptoms
|
3 □
| |||
Patient has 1 symptom
|
2 □
| |||
Patient is asymptomatic
|
0 □
| |||
4.2
|
Symptom intensity
|
Any symptom is severe (equivalent to >6 out of 10)
|
3 □
|
Intensity amended by controllability
|
Any symptom is moderate (equivalent to 4–6 out of 10)
|
2 □
| |||
Any symptom is mild (equivalent to 3 or less out of 10)
|
1 □
| |||
Symptoms are absent
|
0 □
| |||
4.3
|
Psychological distress of patient
|
Significant
|
2 □
| |
Mild to moderate
|
1 □
| |||
Absent
|
0 □
| |||
4.4
|
Psychological distress of parent(s)
|
Significant
|
2 □
| |
Mild to moderate
|
1 □
| |||
Absent
|
0 □
| |||
4.5
|
Psychological distress of siblings
|
Significant
|
2 □
|
Omission
|
Mild to moderate
|
1 □
| |||
Absent
|
0 □
| |||
Domain 5
|
Preferences of patient, family and health professional
| |||
5.1
|
Request by patient and family
|
Patient specifically requests a palliative care approach.
|
4 □ Yes
|
Omission
|
0 □ No
| ||||
Family specifically requests a palliative care approach.
|
4 □ Yes
| |||
0 □ No
| ||||
5.2
|
Preference of health professional
|
You feel that this patient would definitely benefit from a palliative care approach.
|
4 □ Yes
| |
0 □ No
| ||||
Total score:
| ||||
No comments:
Post a Comment